
Advance as a leader and Master the Business of the Life Sciences
Entreprenuership
Leadership Development

Biomedical Engineering

Bioinformatics
By combining the strengths of RIT’s highly regarded Executive MBA program with our first-in-class technical curriculum, the Executive MBA with Life Science Electives program is uniquely positioned to help you put your ideas into action. Merging both traditional EMBA coursework with a focus on the life sciences industry allows students to integrate the core competencies found in business, entrepreneurship, and technology commercialization within life sciences-related disciplines, positioning you as a leader in elevating New York State’s life sciences ecosystem.
Program Cadence
Whether hybrid or online, the executive MBA’s cadence is 2 online courses simultaneously for 6 weeks over 16 months with stand-alone in-person exceptions that include:
- 3-day In-person orientation on RIT campus in ROchester, NY (August 6–8, 2025)
- 4-day Business Simulatino Campus Residence (Mid-May 2026)
- 3-day NYC Life Sciences trip (Early Fall 2026)
- 7–10 day International Trip, October 2026 (Life sciences studnets are welcome to join for experiences, international business class is optional.)
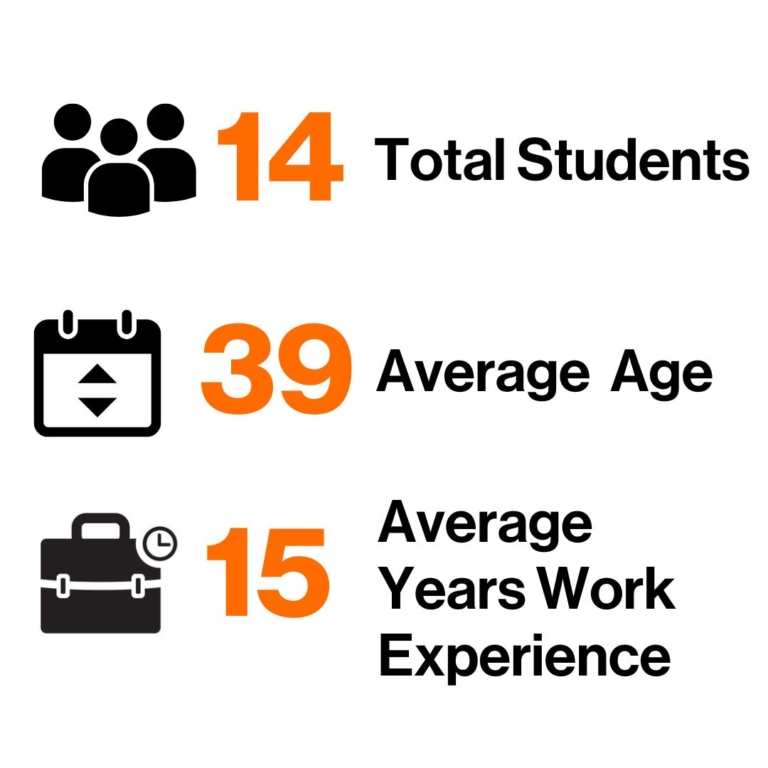
2024 EMBA Class Profile
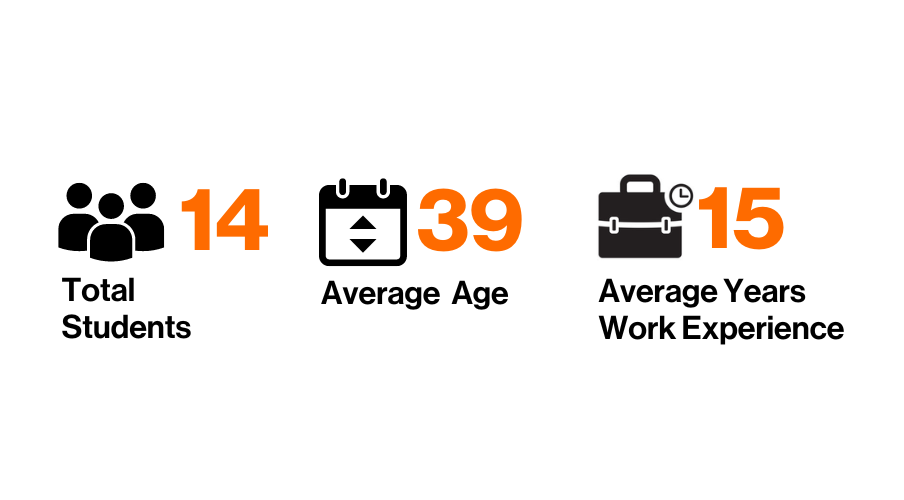

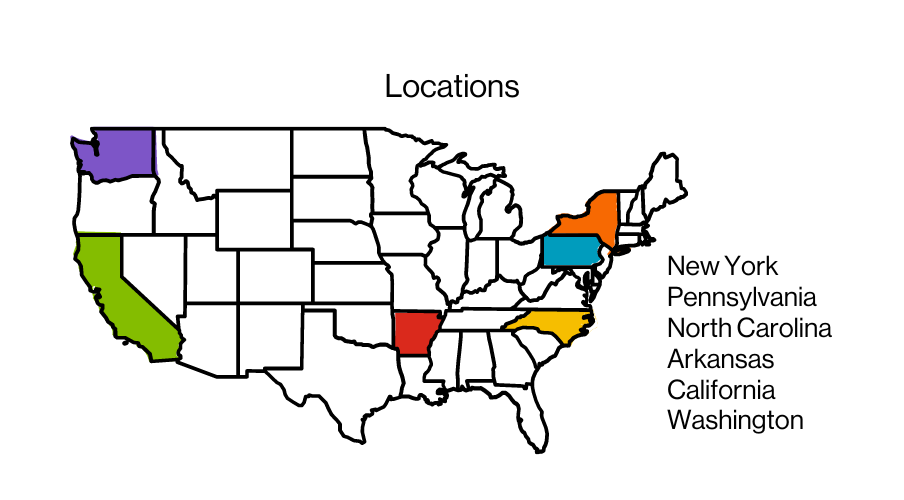
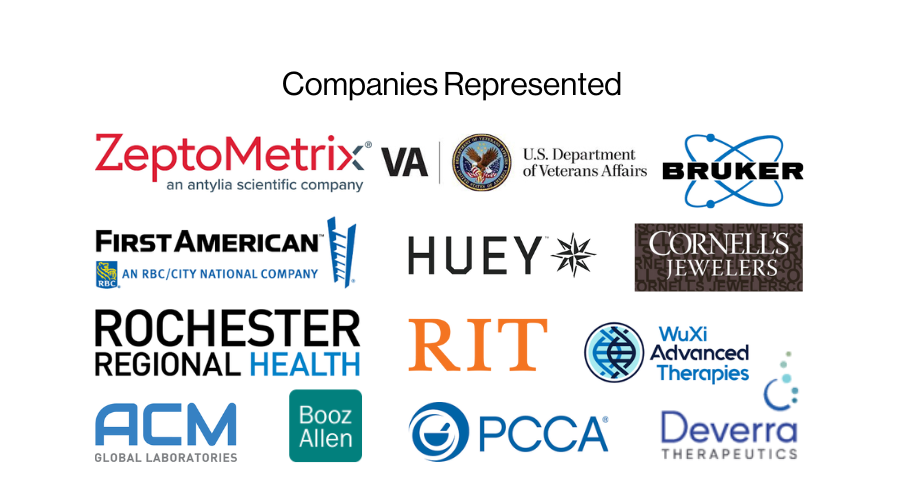
2024 EMBA Class Profile


Scholarship, Aid, & Admission
To demonstrate our commitment to support Life Sciences New York members in achieving their goals through continual education, we are offering an automatic $25,000 executive scholarship to every accepted qualifying member. For additional scholarship award, we offer the following:
$1,000 Early Decision Award for accepted applications completed by June 1, 2025.
Additional scholarship may be available for qualifying nonprofit professionals, entrepreneurs / entrepreneurial minded candidates, and company match for employers that offer tuition benefits.
- Notable achievements (community service, leadership, within or outside work)
- Financial need (availability of company sponsorship / reimbursement, etc.)
- Personal statement: Focusing on how an EMBA will achieve personal and professional goals, and the scholarship question about what you would accomplish if money were no object.
- GPAs may come into consideration
Customized Learning Outcomes
Core business curriculum includes organizational and personal leadership development, strategic thinking, negotiations, and managing tech innovation.
Life sciences electives typically replace the executive leadership, international finance and international business courses.
Their cadence slightly differs from Executive MBA courses as they are taught in partnership with our RIT Colleges of Sciences and Engineering:
BIOL 625 Ethics in Bioinformatics
This course will be focused on individual and organizational responsibilities in bioinformatics research, product development, product commercialization and clinical and consumer genetic testing. Tentative schedule: Fall 2025, approximately 7-15 weeks depending on scheduled format.
BIME Graduate Biodesign
This course is a graduate-level introduction to the biodesign process used for innovating medical technologies. Student teams will apply a needs-based assessment strategy to identify opportunities in a biomedical-related field such as assistive technologies and rehabilitation engineering. Incorporating CAD will culminate in a virtual medical device prototype. Concepts of intellectual property, regulatory considerations, reimbursement, and business models will be introduced. Tentative schedule: Fall 2025, approximately 7-15 weeks depending on scheduled format.
BIME 617 Principals of Biomedical Device Regulations
This course will present the principles and fundamentals of medical device and in vitro diagnostic regulation. The course will cover the history of the FDA and the regulations around food, drug, and cosmetic products. An overview of regulatory pathways, clinical trials, good manufacturing practices, and quality system design will be covered. Comparisons between the US, EU, and other international regulatory bodies will also be discussed. The course will culminate with students developing a clinical trial and regulatory strategy for a new hypothetical medical device. Tentative schedule: Fall 2025, approximately 7-15 weeks depending on scheduled format.
