
Join the next sponsored cohort through RIT Executive Education
Rochester Regional Health offers sponsored enrollment in a 12-month hybrid program for qualifying employees, merging healthcare and business learnings with credits towards a RIT executive MBA
The RIT and Rochester Regional Health alliance aims to improve healthcare quality, cost, and the health of Western New York and the Finger Lakes.

Program Overview, Cadence, and Courses
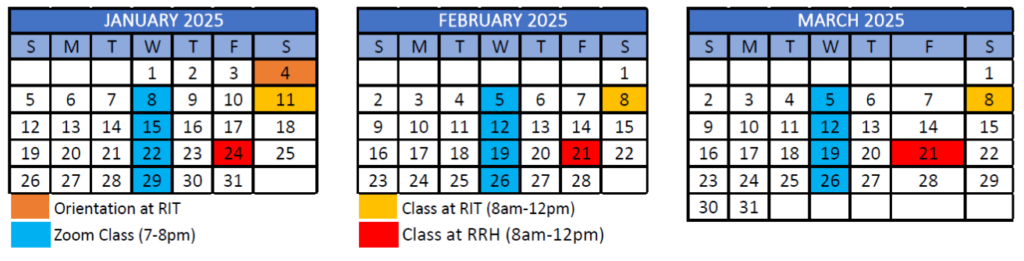
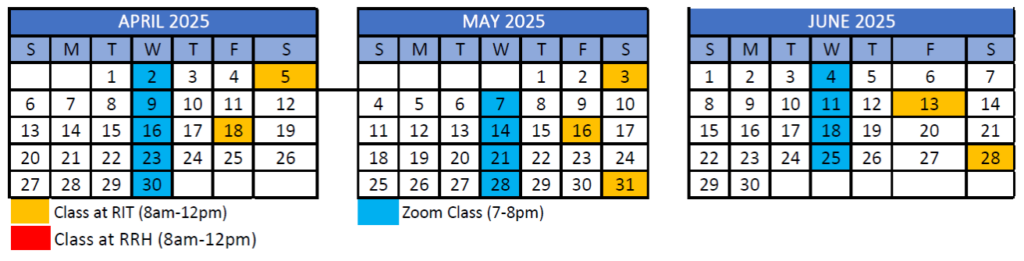
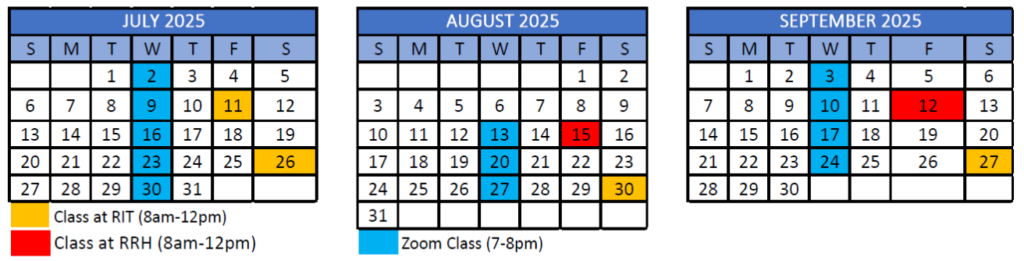
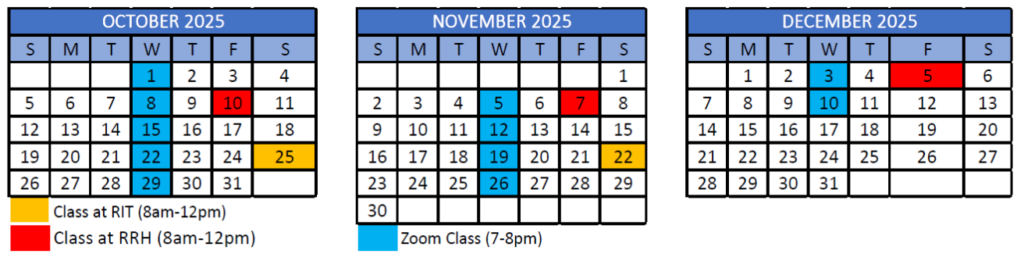
12-month program starts January 2025 and ends December 2025
- Orientation on RIT campus – Saturday, January 4, 2025
- First class meets virtually – Wednesday, January 8, 2025
8 total courses in Hybrid Format
- Each course completed over 6 week blocks, totaling 18 hours of class time with additional work completed outside of classes
- 3 in-person classes per course alternating between the RIT and Rochester Regional Health’s campuses
- 6 live online sessions normally on Wednesdays 7pm-8pm.
Calendar Overview
Tentative program schedule
Course Descriptions
Accounting & Organization Goals
Accounting provides an understanding of how accounting helps organizations achieve their goals. Special emphasis is given to the resolution of controversial accounting issues within the context of a firm’s goals. Topics include standards and practices of financial reporting, financial statements, inventories, long-term assets, bonds and other liabilities, and stockholders’ equity.
Managerial Accounting
Emphasizes identifying and applying the techniques used by managerial accountants to measure the cost of goods and services produced by the firm. The course focuses on understanding how managerial accounting is used to help organizations achieve their goals.
Valuation & Capital Budgeting
The course introduces financial concepts of risk, return and valuation. The main application studied in this course, Capital Budgeting, arises in the corporate setting where managers allocate scarce resources to projects. Basic issues of capital budgeting covered include cash flow estimation and valuation techniques. Advanced issues include sensitivity analysis and the consideration of real options.
Marketing Strategy
A general management perspective on the critical impact of marketing in organizations. Topics include an overview of the marketing process, market research, segmentation, and target markets. The focus is on the process of creating, communicating, and delivering customer value through the marketing mix. The course is structured around the managerially controllable elements of product, price, promotion and distribution, plus the interrelationships of these elements.
Managing Technology, Innovation, & Research
This course deals with the responsibilities and challenges faced by managers responsible for research and innovation within high- technology firms. Topics will include: the critical role of innovation, internal technology assessments, technology transfer, the selection and management of R&D projects, and the identification of and management of disruptive technologies and business models. Particular attention will be given to overcoming systemic barriers to innovation.
This course is customized to focus on disruption in Healthcare.
Managing Negotiations
This course is designed to teach the art and science of negotiation so that one can negotiate successfully in a variety of settings, in day-to-day experiences and, especially, within the broad spectrum of negotiation problems faced by managers and other professionals. Individual class sessions will explore the many ways that people think about and practice negotiations skills and strategies in a variety of contexts. Special emphasis will be on decision-making biases that are often inherent in any negotiation setting and compromise the quality of negotiated agreements.
Strategic Thinking I & II (2 courses)
Strategy examines how firms can achieve superior financial performance through the establishment of a sustainable competitive advantage at the business level. Contemporary theories of strategic management will be discussed and critically examined for their relevance to the problems facing many of today’s managers. Topics include analysis of industry attractiveness, value-chain analysis, core competencies, and business-level strategies
The second course covers implementation, and the interrelations between different corporate divisions. Topics will include related and unrelated diversification, and the various means of engaging in diversification—mergers and acquisitions, joint ventures, and strategic alliances. Contemporary theories of strategic management will be discussed and critically examined for their relevance to the problems facing many of today’s managers.
In the program’s initial cohort, students represented a variety of specialties and departments:
Apply Today
Complete the form below to be considered for the 2025 Cohort. Rochester Regional Health leadership will review and select participants to sponsor for the 12-month executive education program.
Customized Learning Outcomes
Core business curriculum includes organizational and personal leadership development, strategic thinking, negotiations, and managing tech innovation.
Life sciences electives typically replace the executive leadership, international finance and international business courses.
Their cadence slightly differs from Executive MBA courses as they are taught in partnership with our RIT Colleges of Sciences and Engineering:
This course is a graduate-level introduction to the biodesign process used for innovating medical technologies. Student teams will apply a needs-based assessment strategy to identify opportunities in a biomedical-related field such as assistive technologies and rehabilitation engineering. Incorporating CAD will culminate in a virtual medical device prototype. Concepts of intellectual property, regulatory considerations, reimbursement, and business models will be introduced. Tentative schedule: Fall 2025, approximately 7-15 weeks depending on scheduled format.
BIME 617 Principals of Biomedical Device Regulations
This course will present the principles and fundamentals of medical device and in vitro diagnostic regulation. The course will cover the history of the FDA and the regulations around food, drug, and cosmetic products. An overview of regulatory pathways, clinical trials, good manufacturing practices, and quality system design will be covered. Comparisons between the US, EU, and other international regulatory bodies will also be discussed. The course will culminate with students developing a clinical trial and regulatory strategy for a new hypothetical medical device. Tentative schedule: Fall 2025, approximately 7-15 weeks depending on scheduled format.